ALUMINIUM HISTORY
Aluminium was one of the newest metals to be discovered by humans. Aluminium does not occur naturally in its purest form so it was not discovered until the 19th century with developments in chemistry and the advent of electricity. Aluminium has gone on an incredibly interesting journey from a precious metal to the material used virtually in every sphere of human life in just one and a half centuries.Discovery of aluminium

Tomb of Zhou Zhu, a Chinese commander, who died in the early III century A.D. The ornaments were made of an alloy consisting of 85% aluminium.
Humankind came across aluminium long before the metal we know today was produced. The Natural History by Pliny the Elder, a Roman scientist, told the story of a first century craftsman presenting a cup made of an unknown metal looking like silver, but too light to be sliver, to Tiberius, the Roman Emperor.
Alum, an aluminium-based salt, was used extensively in ancient times. Commander Archelaus discovered that wood was practically flame resistant if it was treated using an alum solution; protecting his wooden fortifications against flamed attack. Alum was used throughout Europe from the XVI century onwards: in the leather industry as a tanning agent, in the paper-pulp industry for paper sizing and in medicine, i.e. dermatology, cosmetology, stomatology and ophthalmology.
Aluminium was named after alum, which is called 'alumen' in Latin. This name was given by Humphry Davy, an English chemist, who, in 1808, discovered that aluminium could be produced by electrolytic reduction from alumina (aluminium oxide), but did not manage to prove the theory in practice.
Alum, an aluminium-based salt, was used extensively in ancient times. Commander Archelaus discovered that wood was practically flame resistant if it was treated using an alum solution; protecting his wooden fortifications against flamed attack. Alum was used throughout Europe from the XVI century onwards: in the leather industry as a tanning agent, in the paper-pulp industry for paper sizing and in medicine, i.e. dermatology, cosmetology, stomatology and ophthalmology.
Aluminium was named after alum, which is called 'alumen' in Latin. This name was given by Humphry Davy, an English chemist, who, in 1808, discovered that aluminium could be produced by electrolytic reduction from alumina (aluminium oxide), but did not manage to prove the theory in practice.

Hans Christian Oersted
1777 — 1851
Hans Christian Oersted from Denmark was successful in 1825; however he apparently produced an aluminium alloy with the elements used in the experiments, rather than pure aluminium.
Hans Christian's work was continued by Friedrich Woehler, a German chemist, who set about working from 30 grams of aluminium powder in October 22, 1827. It took another 18 years of continuous experimentation for Friedrich to create small balls of solidified molten aluminium (globules) in 1845.
Hans Christian's work was continued by Friedrich Woehler, a German chemist, who set about working from 30 grams of aluminium powder in October 22, 1827. It took another 18 years of continuous experimentation for Friedrich to create small balls of solidified molten aluminium (globules) in 1845.
Discovery of aluminium ore. In 1821 Geologist Pierre Berthier discovered reddish clay rock deposits in France. The rock was named bauxite after Les Baux, the area where it was found.

Henri-Etienne Sainte-Claire Deville, an outstanding French chemist and technologist, transferred the chemical method of aluminium creation discovered by scientists to industrial application. He improved the Woehler process and produced the first industrial aluminium together with his partners at Charles and Alexandre Tissier's production facility in Rouen (France) in 1856.
200 tonnes
of metal were produced in 36 years (1855-1890) when the chemical method developed by Sainte-Claire Deville was applied
The produced metal resembled silver, it was light and expensive, hence at that time aluminium was considered an elite material intended for ornaments and luxury items. The first aluminium products are considered to be medals made during Napoleon III's reign. Napoleon supported the development of aluminium production and Friedrich Woehler designed a rattle for Crown Prince Louis Napoleon made of aluminium and gold.
However, even then Sainte-Claire Deville understood that the future of aluminium was not just to be associated with jewellery:
However, even then Sainte-Claire Deville understood that the future of aluminium was not just to be associated with jewellery:

"There is nothing harder than to make people use a mew metal. Luxury items and ornaments cannot be the only sphere of its application. I hope the time will come when aluminium will serve to satisfy the daily needs."
Sainte-Claire Deville
French chemist
Sainte-Claire Deville
French chemist
Hall-Héroult process
Aluminium's development changed with the discovery of a more cost-efficient electrolytic production method in 1886. It was developed by Paul Héroult, a French engineer, and Charles Hall, an American student, independently and at the same time. The method involved the reduction of molten aluminium oxide in cryolite. The process demonstrated excellent results, but required an enormous amount of electric power.

Paul Héroult
1863-1914

Charles Hall
1863-1914
Héroult avoided this problem by harnessing the power of the Rhenish Falls in Neuhausen (Switzerland) where the force of the falling water bought the smelters dynamos into operation.
The Swiss Metallurgical Society and Rathenau, a German industrialist, signed an agreement establishing the Aluminium Industry Joint Stock Company with the total value of CHF 10 million in Neuhausen on November 18, 1888. Later it was renamed to the Aluminium Smelter Society and its logo depicted the sun rising from beyond an aluminium ingot. Rathenau's idea was that it should symbolise the dawn of the aluminium industry. Following the Aluminium Smelter Society's creation aluminium production efficiency increased more than 10 times in five years. Just 40 tonnes of aluminium were melted in 1890 in Neuhausen compared with 450 tonnes in 1895.
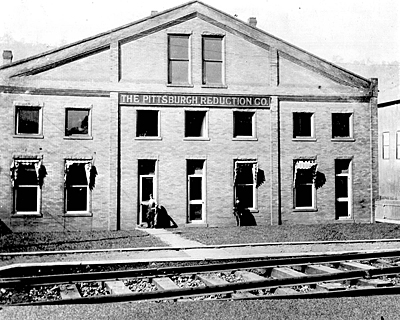
Charles Hall, with the support of his friends, established the Pittsburgh Reduction Company which launched its first smelter in Kensington outside Pittsburgh on September18, 1888. It produced only 20-25 kg of aluminium per day in the first few months, but quickly accelerated to 240 kg daily by 1890.
The Pittsburgh Reduction Company built new smelters in New York State near the new Niagara hydroelectric power station. Smelters are historically built in the vicinity of powerful, cheap and environmentally friendly energy sources, such as hydroelectric power stations, even today. The Pittsburgh Reduction Company was reorganised into the Aluminium Company of America, or more commonly known, Alcoa in 1907.
The Pittsburgh Reduction Company built new smelters in New York State near the new Niagara hydroelectric power station. Smelters are historically built in the vicinity of powerful, cheap and environmentally friendly energy sources, such as hydroelectric power stations, even today. The Pittsburgh Reduction Company was reorganised into the Aluminium Company of America, or more commonly known, Alcoa in 1907.
Karl Joseph Bayer, an Austrian chemist, invented a cheap and feasible alumina (aluminium oxide) production method in 1889 when working in St. Petersburg (Russia) at the Tentelevsky production facility. Going forward, Alumina became the basic raw material for aluminium production. The scientist added bauxite into an alkali solution and heated it in a closed vessel finding that the bauxite dissolved but not completely. Bayer did not find aluminium in the undissolved remains; however he found that entirety of the aluminium in the bauxite was transferred to the alkali solution during the process.
Aluminium production processes used today are based on the Bayer and Hall-Héroult processes.
The aluminium industry was created over several decades. The story of the 'clay silver' came to an end and aluminium became a new industrial metal.
Aluminium production processes used today are based on the Bayer and Hall-Héroult processes.
The aluminium industry was created over several decades. The story of the 'clay silver' came to an end and aluminium became a new industrial metal.
Widespread Use

Aluminium began to be used in various ways at the turn of the XIX and XX centuries which created an incentive for development in a new range of industries.
Alfred Nobel ordered the creation of Le Migron, the first passenger boat to use an aluminium hull, in Switzerland, 1891. Three years later the Scottish shipbuilding yard Yarrow & Co created a 58 metre torpedo boat made of aluminium named Sokol. Sokol was made for the Russian Empire's Navy and accelerated to a speed of 32 knots, a record speed for the time.
In 1894 in New Haven, New York, Hartford Railroad, the American rail company then owned by banker John Pierpont Morgan (J.P. Morgan), started to produce special lightweight passenger railroad cars with aluminium seats. Just 5 years later, Karl Benz presented the first sports car with an aluminium body at an exhibition in Berlin.
Alfred Nobel ordered the creation of Le Migron, the first passenger boat to use an aluminium hull, in Switzerland, 1891. Three years later the Scottish shipbuilding yard Yarrow & Co created a 58 metre torpedo boat made of aluminium named Sokol. Sokol was made for the Russian Empire's Navy and accelerated to a speed of 32 knots, a record speed for the time.
In 1894 in New Haven, New York, Hartford Railroad, the American rail company then owned by banker John Pierpont Morgan (J.P. Morgan), started to produce special lightweight passenger railroad cars with aluminium seats. Just 5 years later, Karl Benz presented the first sports car with an aluminium body at an exhibition in Berlin.
Aluminium statue of Anteros, an Ancient Greek god, appearing in Piccadilly Circus, London, in 1893. At almost two and a half metres high, the statue became the first large piece of art made from aluminium. Several decades earlier, a fireplace clock or figurines in offices were believed to be a piece of luxury available only for high society.


Still, aluminium used for aviation was the real revolution, which is where the name 'winged metal' was born. Inventors and aviators around the world work towards the development of controlled aerial vehicles during this period.
On December 17, 1903, Wilbur and Orville Wright, American aircraft designers, flew a controlled aerial vehicle, the Flyer-1, for the first time in human history. They originally tried to use a car engine to propel the aeroplane, but it turned out to be too heavy. Therefore, a completely new engine with aluminium parts was developed specially for the Flyer-1. A light, 13-hp motor made allowed the first ever plane to become airborne with Orville Wright at the helm for 12 seconds, flying 36.5 metres. The brothers performed another two flights, 52 and 60 metres, at a height of about 3 metres above ground level.
On December 17, 1903, Wilbur and Orville Wright, American aircraft designers, flew a controlled aerial vehicle, the Flyer-1, for the first time in human history. They originally tried to use a car engine to propel the aeroplane, but it turned out to be too heavy. Therefore, a completely new engine with aluminium parts was developed specially for the Flyer-1. A light, 13-hp motor made allowed the first ever plane to become airborne with Orville Wright at the helm for 12 seconds, flying 36.5 metres. The brothers performed another two flights, 52 and 60 metres, at a height of about 3 metres above ground level.
Duralumin, a key aluminium alloy, was invented in 1909. It took seven years for Alfred Wilm, a German scientist, to create it, but it was worth the years of effort. Duralumin, with addition of copper, magnesium and manganese was as lightweight, as aluminium, but significantly exceeded it in strength, hardness and elasticity meaning it quickly became the main material used in aviation. The hull of the first all-metal plane, the Junkers J1, developed in 1915 by a world aircraft industry pioneer, the famous German aircraft designer Hugo Junkers, was made from Duralumin.

When the world entered a period of war, aviation played a crucial and sometimes decisive part. As duralumin was used in military technology initially, its production method was classified.
Meanwhile, aluminium gained uses elsewhere. Aluminium began to be used for the mass production of houseware that quickly and almost completely replaced copper and cast iron utensils. Aluminium frying pans and saucepans are light, warm and cool quickly and do not rust.
Meanwhile, aluminium gained uses elsewhere. Aluminium began to be used for the mass production of houseware that quickly and almost completely replaced copper and cast iron utensils. Aluminium frying pans and saucepans are light, warm and cool quickly and do not rust.

Robert Victor Neher invented the method used for continuous aluminium rolling foil production in Switzerland in 1907. He launched the world's first foil rolling mill in 1910. A year later Tobler started to use foil for chocolate packing, still used to this day, even the famous Toblerone bar is wrapped in it too!
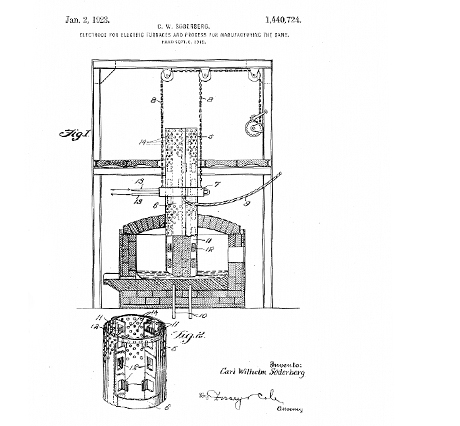
Another critical moment for the aluminium industry began in 1920, when a group of scientists under the leadership of Soderberg from Norway invented a new aluminium production process that made the Hall- Héroult method much cheaper. Previously, pre-baked coal blocks were used as anode cells during reduction; they were consumed quickly and so the use of new ones was required constantly. Soderberg solved this problem with the help of a continuous electrode. It is formed in a special reduction chamber from a coke and tar paste and added to the upper hole of the electrolyte cell when required.
The Soderberg process was quickly adapted in aluminium production throughout the world and resulted in output increase. The USSR had no aluminium industry at that time and added this to its armoury.
Thereafter, the application of electrolyte cells with pre-baked anode cells became more preferable due to the lack of resin emissions and lower power consumption. In addition, one of the main advantages of electrolytic cells with pre-baked anode cells was the possibility to increase amperage and therefore capacity.
The Soderberg process was quickly adapted in aluminium production throughout the world and resulted in output increase. The USSR had no aluminium industry at that time and added this to its armoury.
Thereafter, the application of electrolyte cells with pre-baked anode cells became more preferable due to the lack of resin emissions and lower power consumption. In addition, one of the main advantages of electrolytic cells with pre-baked anode cells was the possibility to increase amperage and therefore capacity.
As early as 1914, Nikolai Pushin, a Russian chemist, wrote: 'Russia consumes 80,000 puds of aluminium annually, but does not produce a single gram of this metal and buys all aluminium abroad'.
In 1920, despite the continuing civil war, Russia's leaders finally understood the colossal amount of power required to generate industrial growth and development within their enormous territory. In light of this, the Russian government initiated GOELRO (the State Committee for Electrification of Russia), which involved the construction of hydroelectric power stations ("HPS") on Russian rivers. It was decided to build aluminium smelters next to water cascades for them to have consumers for the generated power. In so doing aluminium was employed for both military and civil needs. The first Volkhovskaya HPS was commissioned in the Leningrad region in 1926. The Volkhovsky aluminium smelter that produced its first metal in 1932 was built next to it. By the beginning of World War II Russia had two aluminium smelters and one alumina refinery. Another two aluminium smelters were built during the war.
In 1920, despite the continuing civil war, Russia's leaders finally understood the colossal amount of power required to generate industrial growth and development within their enormous territory. In light of this, the Russian government initiated GOELRO (the State Committee for Electrification of Russia), which involved the construction of hydroelectric power stations ("HPS") on Russian rivers. It was decided to build aluminium smelters next to water cascades for them to have consumers for the generated power. In so doing aluminium was employed for both military and civil needs. The first Volkhovskaya HPS was commissioned in the Leningrad region in 1926. The Volkhovsky aluminium smelter that produced its first metal in 1932 was built next to it. By the beginning of World War II Russia had two aluminium smelters and one alumina refinery. Another two aluminium smelters were built during the war.
Aluminium was widely used in the aviation, shipbuilding and automotive industries during that time and started its progress in civil engineering. The Empire State Building, which was the highest building worldwide until 1970, was built in 1931. It was the first building where aluminium was widely employed in construction, both in the basic structures and in the interior. World War II changed the complexion of the basic aluminium market bringing aviation and the manufacture of tank and automotive engines to the forefront. The war pushed the countries fighting Germany to increase the use of their aluminium production capacities. Designs of aircraft were improved and the new aluminium alloys were developed with them. Joseph Stalin, the leader of the USSR, wrote to Franklin Roosevelt, the President of the USA, in 1941: 'Give me 30 thousand tonnes of aluminium, and I will win the war'. The smelters returned to civil products after the end of the war.
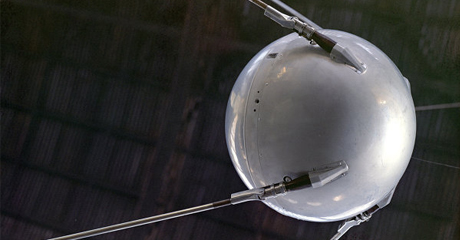
The human race took a leap into space in the middle of the XX century. The use of aluminium was critical and therefore triggered the aerospace industry to become one of the key spheres. In 1957, the USSR launched the first artificial satellite into orbit. The satellites hull consisted of two separate aluminium semi-spheres joined together. All subsequent space vehicles were produced using aluminium.
The aluminium can, an iconic product that became one of the largest aluminium commodities, the symbol of environmental friendliness and a focal point in the field of art and design, emerged in the USA in 1958. The cans invention was shared between Kaiser Aluminium and Coors. Coors was not only the first company to sell beer in aluminium cans but also organised the collection of empty cans using a recycling system. Coca-Cola and Pepsi started to sell their drinks in aluminium cans in 1967.

In 1962, the legendary race car driver Mickey Thompson and his race car the 'Harvey Aluminium Special' made from aluminium alloys became a sensation at the Indianapolis 500. Despite the fact that his car has 70 HP less power than his competitors, Thompson came eighth in the qualification and ninth in the race. As a result, his team got the Mechanical Achievement Award for breakthrough in race car design.
Aluminium was the key material used in Shinkansen, the famous high-speed train and prototype of all modern trains of that type. Modern high speed trains were launched in Japan two years later. The train ran between Tokyo and Osaka and travelled 515 km in 3 hours and 10 minutes, a top speed of 210 km/hour. The USSR stepped up the tempo of their aluminium production through the use of hydroelectric plants in Siberia and in doing so, became the world's aluminium market leader. In the mid-1960s two giants of the aluminium industry, the Bratsk and Krasnoyarsk aluminium smelters, which each had the capacity of 1 million tonnes of metal per year, were commissioned in Siberia. These production facilities remain the largest in the world.
Aluminium was the key material used in Shinkansen, the famous high-speed train and prototype of all modern trains of that type. Modern high speed trains were launched in Japan two years later. The train ran between Tokyo and Osaka and travelled 515 km in 3 hours and 10 minutes, a top speed of 210 km/hour. The USSR stepped up the tempo of their aluminium production through the use of hydroelectric plants in Siberia and in doing so, became the world's aluminium market leader. In the mid-1960s two giants of the aluminium industry, the Bratsk and Krasnoyarsk aluminium smelters, which each had the capacity of 1 million tonnes of metal per year, were commissioned in Siberia. These production facilities remain the largest in the world.
Increased aluminium production volumes globally and demand for the metal resulted in aluminium becoming an exchange commodity in the 1970s. In 1978, the exchange trade for aluminium contracts started on the London Metal Exchange ("LME"), the oldest exchange in the world, established in 1877. Since then the price for primary aluminium became uniform all over the world and was formed during exchange trade on the LME.

Aluminium production was growing steadily worldwide and reached 19 million tonnes by the beginning of the 1990s. The role of China started to become more important with the centre of the world's production gradually drifting to its territory. Domestic aluminium production in China at the time did not exceed 900 thousand tonnes, but it was growing rapidly, supplying their own needs. The aluminium production facilities in Russia reached 3.5 million tonnes annually, but the country lived through the dissolution of the Soviet Union and subsequent economic collapse. As Russia began to change their economic model aluminium production growth slowed.

China's production grew past Russia's in 2002 exceeding 4.3 million tonnes. 26 million tonnes of aluminium were produced worldwide at that time. Hereafter, aluminium production in China grew at priority rates. It reached almost 10 million tonnes by 2006, one third of total global production volumes.
China utilised all produced aluminium internally. Turnaround of the metal and other materials was so great, that they had their own commodity exchanges, which were merged into the Shanghai Futures Exchange ("SHFE") in 1999.
At the same time, China ramped up its production at a high environmental price. More than 90% of power which is used for aluminium production is generated from coal power stations. For comparison, the situation is reversed in Russia and about 90% of aluminium production is powered by hydraulic energy.
China utilised all produced aluminium internally. Turnaround of the metal and other materials was so great, that they had their own commodity exchanges, which were merged into the Shanghai Futures Exchange ("SHFE") in 1999.
At the same time, China ramped up its production at a high environmental price. More than 90% of power which is used for aluminium production is generated from coal power stations. For comparison, the situation is reversed in Russia and about 90% of aluminium production is powered by hydraulic energy.
Countries in the Middle East also started to play a significant part in the aluminium industry too. Having access to cheap oil and associated natural gas, aluminium producers there had a source of cheap but environmentally harmful power. They also ramped up their production vigorously and today rank among the world leaders in production of the winged metal.Challenges for the world aluminium industry began in 2008 during the global financial crisis. The aluminium industry was confronted by an overproduction crisis for the first time in its history as a result of the stock market collapse and as a consequence experienced a 50% drop in the price of aluminium. Millions of tonnes of aluminium accumulated in storage facilities worldwide. Exchange traders showed interest in them: financial deals with the metal became a profitable investment.
The crisis of 2008-2009 led to large-scale closures of smelters belonging virtually to all Western aluminium companies. At the same time, metal production continued to grow throughout the world. Producers in China and the Middle East moved in the opposite direction and ramped up production.
In 2013, the global aluminium industry made fresh gains with production exceeding 50 million tonnes. Further industry development is intimately connected with the growth of consumption as a result of further urbanization and industrialization globally. Aluminium will be more actively replacing heavier steel in the automotive industry and a more expensive copper in electrical engineering. According to forecasts, the demand for aluminium will exceed 80 million tonnes by 2023.Technical progress in the industry will continue and basic processes will improve creating new alloys. Today the Soderberg process is being improved as well the development of the inert anode and cell operating capacity increases in terms of amperage. All these developments are aimed at increasing environmental and economic efficiency. At the same time, new alloys are being developed so that aluminium can find new application niches. As you can see, the history of aluminium's development and the industry is very unique. This metal has remained an enigma for millennia and became the most popular structural material in just a century
Comments
Post a Comment